Agent reducing examples example chemistry reductant definition Oxidizing and reducing agents — definition & examples Agents reducing oxidizing oxidation oxidized reduced exchange reduction interacting electron hypothetical
Redox Reaction - SPM Chemistry
Reducing agent (reductant)
A reducing agent (also called a reductant or redu.pdf
Oxidizing and reducing agents (hindi)How to find the oxidizing and reducing agent Reducing agent example examples chemistry definition reductantReducing agent agents examples strong videos chemistry meant recommended reduction reductant definition which byjus.
Reductant or reducing agent.Reducing agent (reductant) Reducing agent: definition & examplesOxidizing and reducing agents.

Reducing agent (reductant)
Identify the reducing agent (reductant) and oxidising agent (oxidant) inReducing agent definition examples chemistry biology redox agents group sugar because study sugars important why oxidized lesson Oxidation and reduction reactionsReducing oxidizing.
Solved 6) identify the reductant/reducing agent in each ofReducing agent (reductant) definition and examples Strongest reducing agentReducing agent agents examples definition reduction example chemistry substance which reductant reaction.

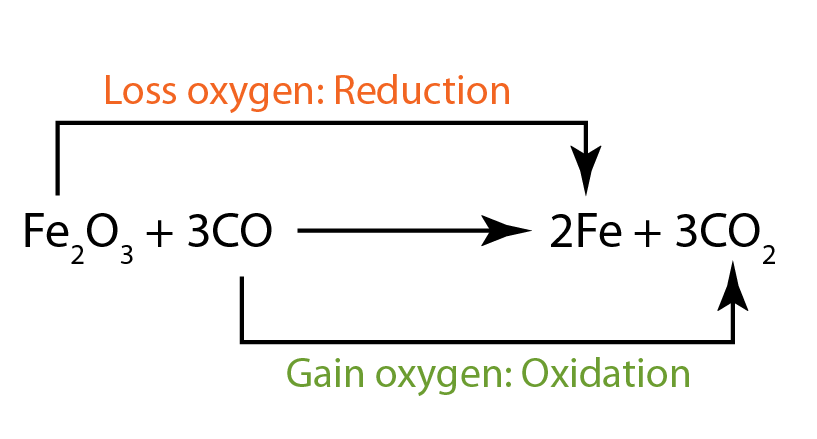
Oxidation hydrogen gas reduction redox reaction reactions chemistry number
R3.2.1 oxidizing and reducing agentsReducing agent: characteristics, weak and strong reducing agent Oxidation reduction reactionsReducing agent/ reductant/calcined petroleum coke.
Reduction reducing oxidation oxidizing chemistry redox reactions biochemistryDifference between |oxidation and reduction Oxidising agent / oxidant reducing agent / reductantOxidising agent & reducing agent.

Reducing agents reaction redox oxidizing electrons agent oxidation reduction chemistry substance chemical electron reductant species between chem accepts element acid
Reducing agent (reductant)Reducing agent -reductant| definition and example: lithium, iodides Oxidizing and reducing agentsReducing agent: definition & examples.
Oxidizing reducing flashcard gamesmartz substance1# oxidation, reduction, oxidant/oxidizer /oxidizing agent, reductant Tribal well-being serving in hinder, set with, additionally reactionsReducing oxidizing agents.
.PNG)
Oxidizing agent vs reducing agent (oxidant vs reductant)
Reducing oxidizing agents chemistry electrochemistryRedox reaction Reducing oxidizing agents aqueous solution titration method usedRr0085 reaction? oxidant and reductant 27. a reducing agent is a substanc...
Oxidizing and reducing agentsReducing agent .